4. "rMMS" and regular microseeding 
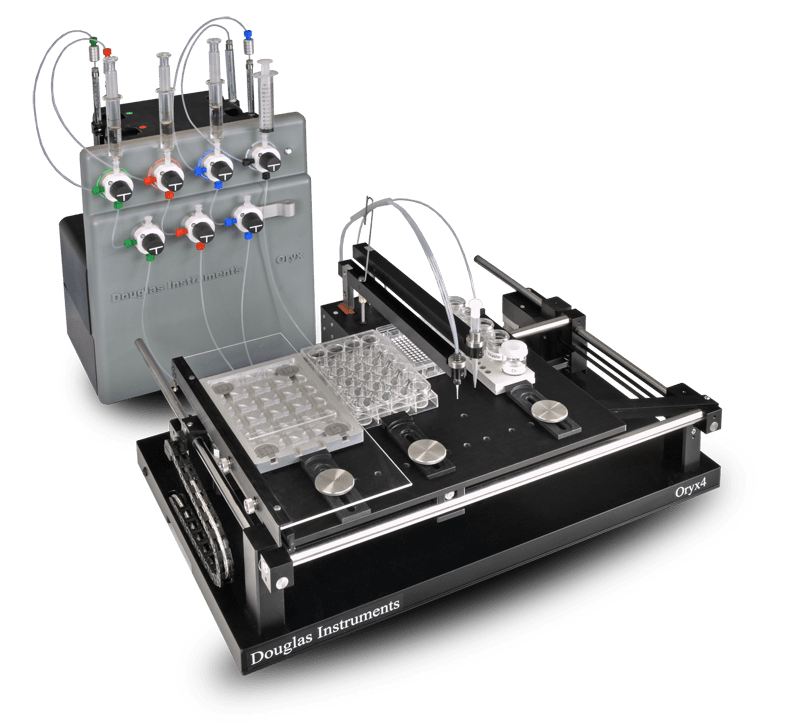
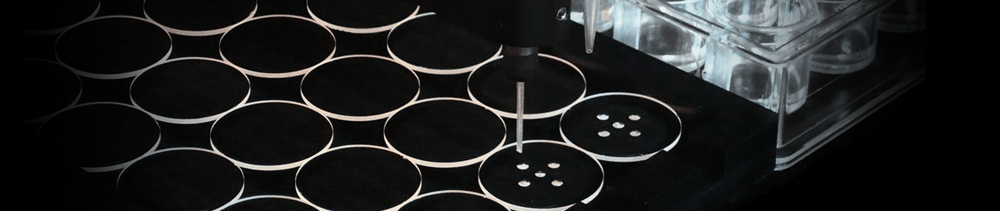
Since all Oryx systems use contact dispensing (the tip always touches
the plate) they give very reliable dispensing even when suspensions of
solid particles are used. This makes them ideal for microseeding
experiments. The technique of adding crystal
seed-stock to random
screens (rMMS) is a significant breakthrough in protein crystallization
that is very effective. For example, one industrial group
used the
method to solve 38 out of 70 structures generated in a four year period,
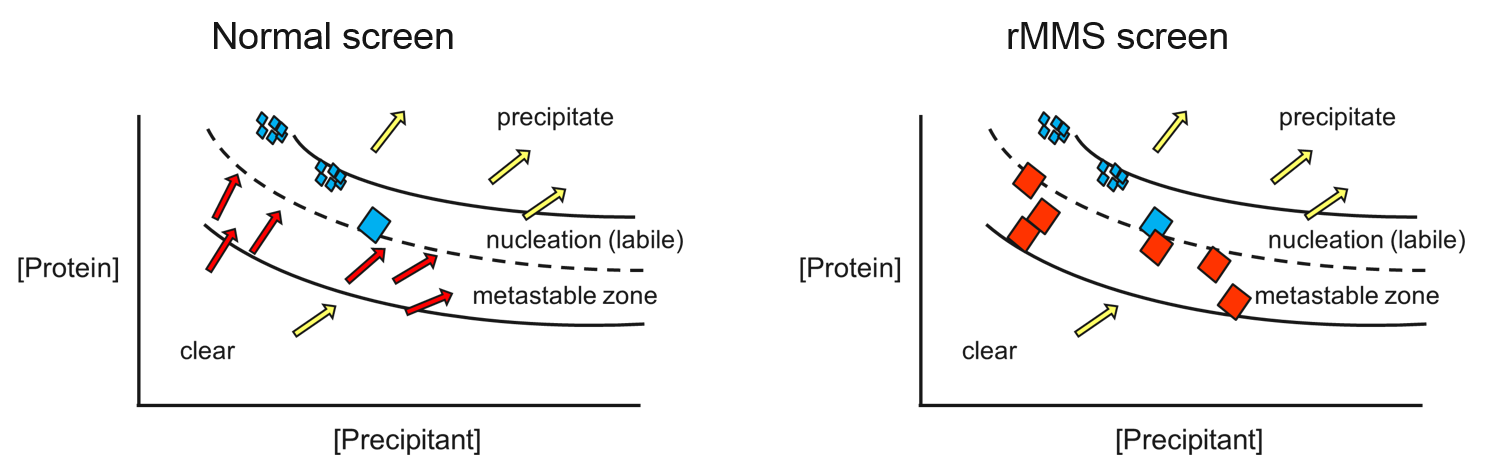
finding particular success with antibody complexes. rMMS not only
produces more hits, it also typically generates better-diffracting
crystals – because crystals are more likely to grow in the metastable
zone of the protein’s phase diagram (see below).
Note also that in cases where only one or a few crystals are obtained in
screening experiments, the seed stock that can be made is very valuable
– often more valuable than the protein sample. It is therefore a great
advantage to be able to use the smallest possible sample of seed stock.
Using any robot from the Oryx range, seeding can be performed in a whole
96-well plate using only 1.5 µl of seed stock. This is particularly
helpful for membrane protein crystallization projects because membrane
protein crystals are often unstable and it is helpful to make seed
stocks without diluting the original mother liquor.
 Videos about rMMS : (1)
Introduction and theory. (2)
How to make Seed Stock for rMMS
Videos about rMMS : (1)
Introduction and theory. (2)
How to make Seed Stock for rMMS
5. Minimal protein or seed-stock wasted 
Contact dispensing has another advantage: almost no protein remains in
the tip at the end of the experiment. Moreover, since only one
(multi-channel) tip is used, all of the protein for an experiment can be
placed in a single PCR tube, which also reduces waste. When they have
enough protein, most users set up 300 + 300 nl drops. For a 96-well
plate this requires only 29.4 µl of protein, i.e. only 0.6 µl is wasted.
If your pipette is accurate, there is no need to put more than the
specified amount into the tube!
Similarly if 10 nl of seed stock is added to each drop, only about 1.5
µl of seed stock is required for a whole 96-well crystallization plate.
(It is helpful to dispense around 5 µl of screen solution on top of the
seed stock.)
6. Simple optimization experiments 
All Oryx systems can carry out simple optimization experiments using three different approaches.
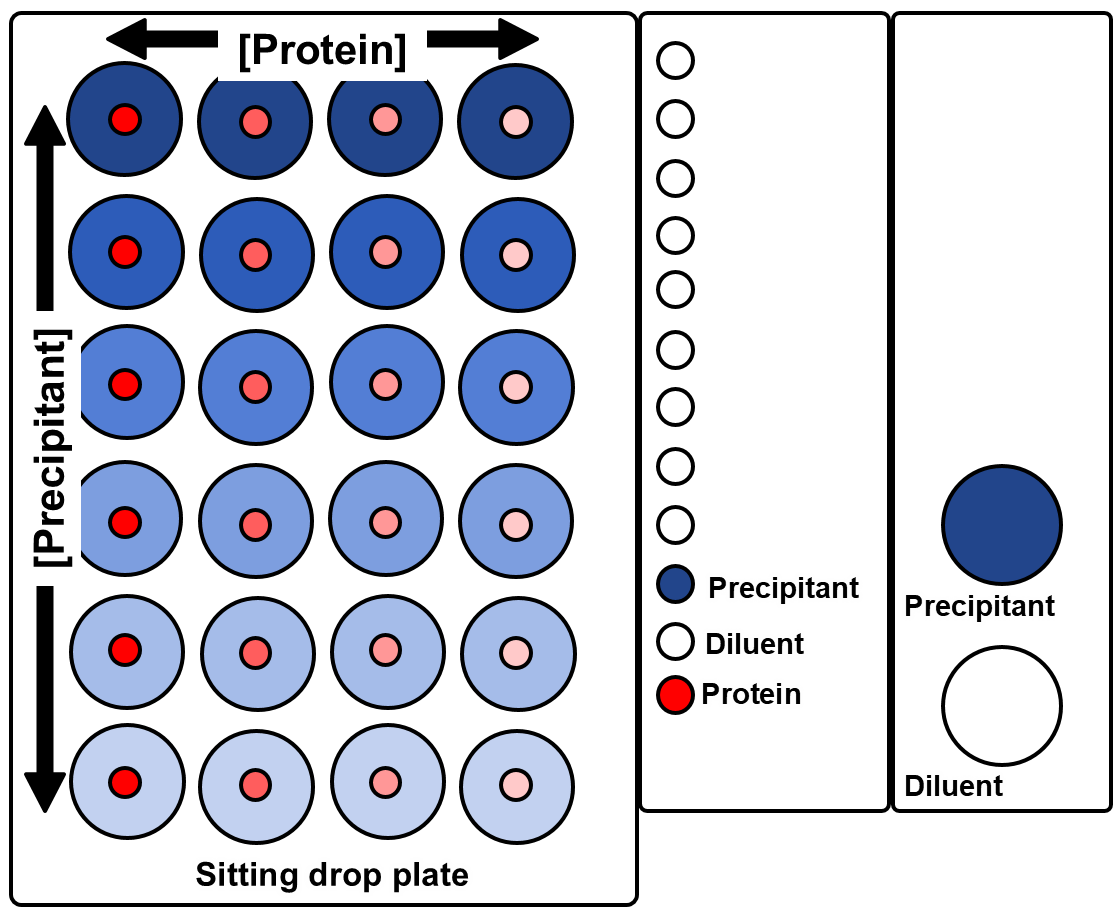
Simple 2D gradients 
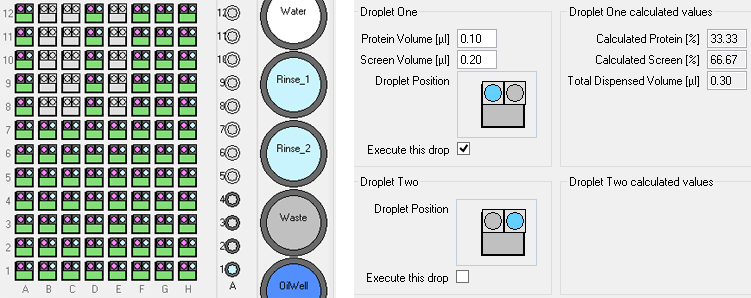
The standard screening software allows users to define simple 2D grids for sitting drop, *hanging drop and *microbatch-under-oil experiments (*Oryx4 and 8 only).
These experiments use three or four ingredients, the user specifies the minimum and maximum concentrations to be dispensed to the corners of a rectangular grid.
The software interpolates linearly between those conditions. (The user interface does not show the concentrations in intermediate wells, but the volumes dispensed to each well are shown in a report file.)
For vapor diffusion experiments, the robot dispenses ingredients to the reservoir well first, then loads protein and other precious solutions such as seed stock before dispensing all ingredients to the drop well simultaneously.
Note: Oryx Nano robots do not have a large volume dispensing channel and therefore can only dispense smaller volumes of ingredient solutions to the reservoir well.
Oryx Nano robots typically dispense a total volume of up to 20 µl to each reservoir well. Oryx4 and 8 robots typically dispense a total volume of 20 - 500 µl to each reservoir well.
(A more sophisticated approach to optimization that includes gradient experiments, multivariate optimization and reservoir filling with a greater number of ingredients is available with the Oryx8 – see below.)
 Videos of 2D grid experiments:
Videos of 2D grid experiments:
(1)24 Well Hanging Drop.
(2)96 Well Sitting Drop.
(3)Microbatch-Under-Oil 2D Gradient.
Cross-Matrix (additive) optimization 
The systems' powerful “combinatorial optimization” approach allows a
different additive or seed-stock to be added to each row. Each additive
is picked up from the corresponding PCR tube on the right of the table
(A1, A2 etc. on the diagram below). By arranging e.g. precipitants in
columns (P1, P2 etc.), different combinations of precipitants and
additives can be tested very quickly. This is useful for reshuffling the
ingredients of several hits, so that ingredients that are not helpful
can be eliminated quickly, and trends can be identified. For example,
certain ingredients may encourage the formation of crystals with certain
morphologies.
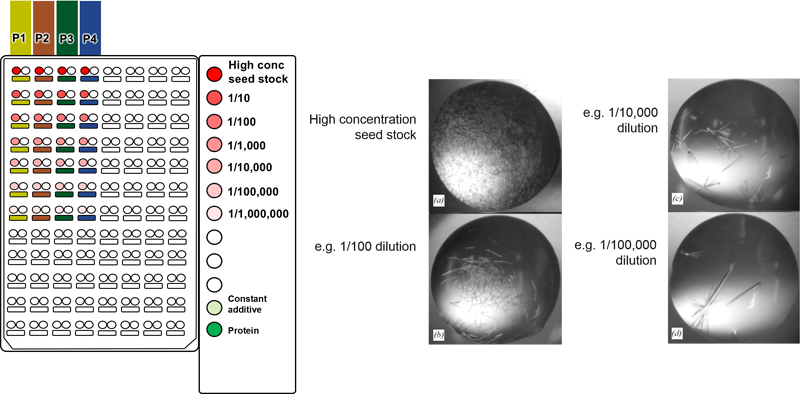
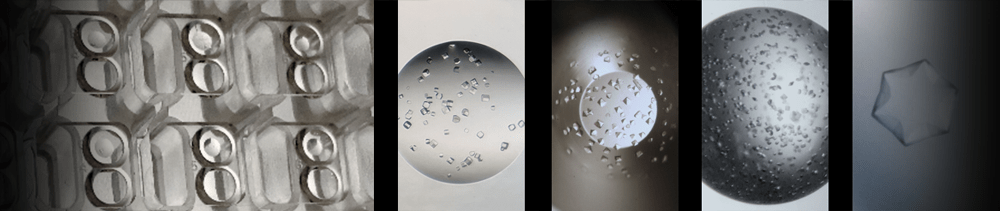
The combinatorial approach can also be used to systematically identify the appropriate dilution of a seed stock in a single experiment. We recommend using a highly concentrated seed stock
for routine rMMS screening, however this can result in showers of small crystals. It is often possible to optimize these conditions by diluting the seed stock to get around 5
crystals per drop (experiment with thermolysin shown above). For example, different concentrations of seed stock
could be placed in the PCR loading tubes shown 1 - 1E-6 dilution above. Four different conditions
could be placed into the four columns labeled P1 to P4 above.
This is a very effective way to get a really reliable supply of crystals
for data collection and soaking experiments.
 Video about
Cross-Matrix Optimization
Video about
Cross-Matrix Optimization
Additive scatter optimization 
Scatter up to 5 additives (e.g. seed stocks) evenly distributed across a vapor diffusion plate. The vapor diffusion plate would typically be pre-prepared
with a 2D gradient of precipitant against salt or precipitant against pH. The robot will then distribute up to 5 additives in a
pattern across the 2D gradient. This tests the additives across a range of concentrations.
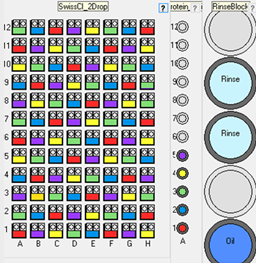
This experiment would normally be used for testing up to 5 dilutions of seed stock. It could also be used to test other additives or protein concentrations.
Features of the Oryx4 and Oryx8 systems only:
7. Hanging drop experiments 
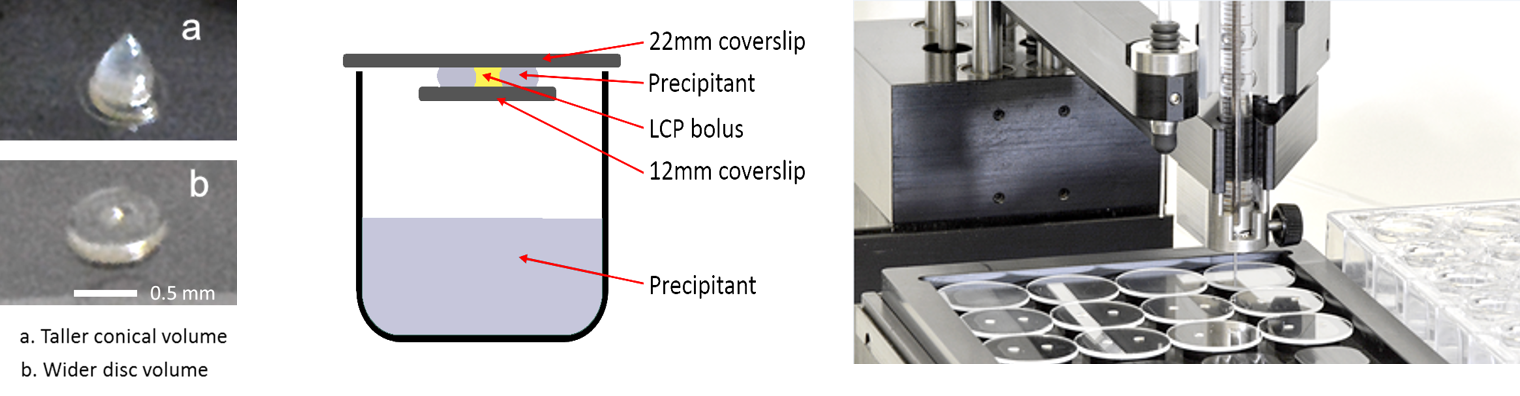
Oryx4 and Oryx8 can set up experiments with 22 mm, 18 mm and 12 mm cover slides as well as 15-well
EasyXtal plates, with up to 5 drops on each
cover slide. Volumes can range from 100+100 nl up to 8+8 µl (assuming 24 drops). You can also add one additive
to each drop with Oryx4, and up to five additives with Oryx8. This is very useful for leads that
are picked up in random microseeding experiments, where it may be necessary to add e.g. diluted seed stock to get crystals.
For example, in the experiment shown (below below), one drop has seed-stock, protein, reservoir solution; the second drop has no seed but
a little extra protein, while the third drop has no seed but a little extra reservoir solution.
You have to transfer the cover slides onto the reservoir tray by hand. After a few drops the experiment can be paused to allow the transfer.
Oryx8 can set up the reservoirs for optimization experiments, including 2D grids and multivariate experiments (see below). The hanging
drop capability is not available for the OryxNano.

 Videos about hanging drop experiments: (1)
2D gradient - 24 Well Hanging Drop. (2)
24 Well Hanging Drop
Videos about hanging drop experiments: (1)
2D gradient - 24 Well Hanging Drop. (2)
24 Well Hanging Drop
8. Additive screening 
Additive experiments are a well-known and effective approach to
optimization. Simply by adding a set of potential ligands or another
screen to a hit condition, crystals can often be optimized. Since they
have larger Plate Loaders, the Oryx4 and Oryx8 systems can accommodate
an extra 96-well plate containing additives to the right of the target
plate. The tip picks up e.g. 100 nl samples from the additive plate and
dispenses them along with protein and reservoir solution to the drops.
For initial screening relatively large volumes of additive are often
used, for example 300 nl (protein) + 100 nl (reservoir) + 200 nl
(additive). For example, these volumes could be used for an initial
screen with the Silver Bullet screen by Hampton Research. For final
optimization, smaller volumes of additive are often used, for example
300 nl (protein) + 200 nl (reservoir) + 100 nl (additive).
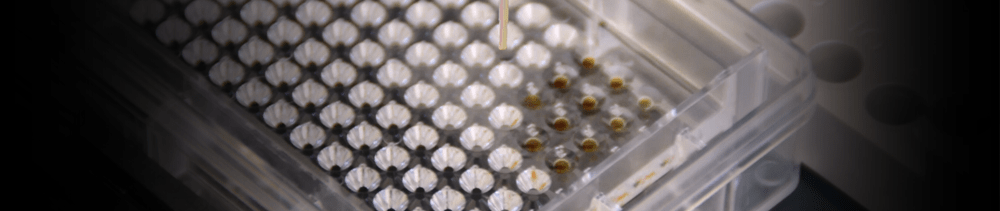
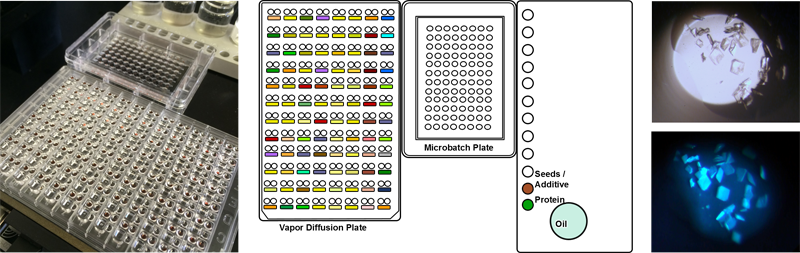
9. Microbatch-under-oil 
Microbatch is a very simple approach to crystallization. Small samples
of protein (100 nl to 5.0 µl) are mixed with stock solutions in small
drops, and covered with oil to prevent evaporation. For screening
experiments it helps to use a 50:50 mixture of paraffin oil and silicone
oil. The silicone allows slow evaporation over about a month, which
gives a scanning effect across the phase diagram of the protein. For
optimization, pure paraffin oil can be used, which reduces evaporation
to a minimum. Studies have shown that microbatch finds as many or
slightly more hits that vapor diffusion, but the main advantage is that
(for reasons that may not be well-understood) certain proteins
crystallize much more effectively in microbatch than other methods. Microbatch can
help to protect sensitive proteins such as membrane proteins and
anaerobically-produced proteins because it reduces the oxidation and
gives thinner skins on the surfaces of drops.
A full set of experiment scripts for microbatch are available, including screening,
additive screening, 2D gradients and MMS microseeding. Oil is automatically dispensed onto the aqueous drop covering it immediately.
Using the Combined MB-VD experiment
it is possible to dispense two screening methods at the same time. It is also possible to dispense different drop ratios or add seed-stock or additive to any experiment or drop.
 Videos about microbatch under oil experiments: (1)
Optimization. (2)
Screening (combined sitting drop and microbatch)
Videos about microbatch under oil experiments: (1)
Optimization. (2)
Screening (combined sitting drop and microbatch)
|